Trial results suggest advancements in treatment for female incontinence
Highlights from three abstract presentations on Friday.
Investigators revealed findings to impact the treatment of patients with urologic conditions in Friday’s abstract session, “PD06-01: Urodynamics/Lower Urinary Tract Dysfunction/Female Pelvic Medicine: Female Incontinence: Therapy I.”
Highlights from three of the 12 abstract presentations included a look at emerging cell therapy, refractory incontinence and exercise.
Emerging cell therapy for urologic indications
Melissa R. Kaufman, MD, PhD, FACS, presented results of “A Double-blind, Randomized, Controlled Trial Comparing Safety and Efficacy of Autologous Muscle Derived Cells for Urinary Sphincter Repair (AMDC-USR) with Placebo (PBO) in Women with Stress Urinary Incontinence (SUI).” Dr. Kaufman is principal investigator and chief, division of reconstructive urology and pelvic health, at Vanderbilt University Medical Center in Nashville. The Phase III clinical trial investigated the safety and efficacy of the intrasphincteric injection of a single dose of 150 × 106 autologous muscle-derived cells (AMDC-USR) for urinary sphincter repair versus placebo in women with stress urinary incontinence (SUI).
“AMDC-USR is a unique technology as a biologic for stress incontinence treatment. It’s targeted for delivery to the external sphincter,” Dr. Kaufman said.
Administering AMDC-USR is a minimally invasive in office procedure. “The mechanism is hypothesized to engraft to the muscle at the injection site, forming new striated muscle and improving muscle function,” Dr. Kaufman said.
The randomized, placebo-controlled Phase 3 study enrolled 297 women who were randomized 2:1 to receive cellular injection or placebo and stratified by prior surgeries for stress incontinence episode frequency severity of less than 10 or greater than 10 over a three-day diary. Of the 297 women, 199 received the cellular product, and 98 received placebo. Subjects who were originally randomized to placebo could opt to receive open label intrasphincteric injection of 150 × 106 AMDC-USR at 12 months. At one year follow-up, 97 placebo patients opted for the open-label study; all were followed for two years.
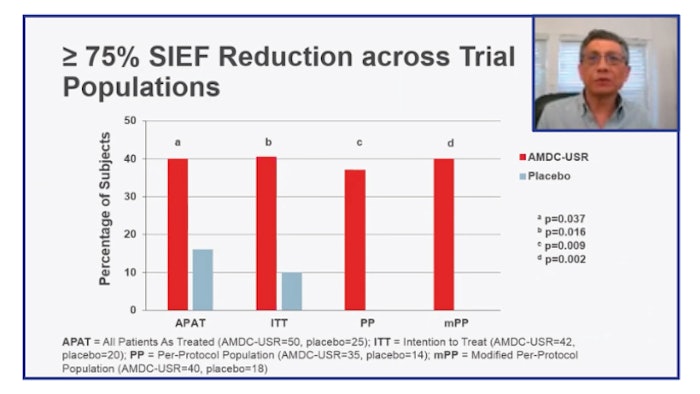
The primary endpoint of greater than 50% stress incontinence episode frequency reduction (SIEF) at 12 months was not discriminatory between placebo due to exceptional variability in the placebo effect. “At greater than 75% SIEF reduction, we did see meaningful differences between the cellular arm and the placebo arm, particularly in patients who had prior surgical interventions and those with a higher number of leaks at baseline,” Dr. Kaufman said.
The correlation between the change in incontinence episode frequency and quality of life was significant, particularly for patients who had a greater than 75% reduction in their stress incontinence episode frequency. For women with SUI, “a single injection of the adult muscle-derived cells is a safe and durable treatment through 2 years,” Dr. Kaufman said, which is consistent with prior data from randomized, double-blind studies.
Helping women with refractory incontinence after surgery
What happens to women who’ve had previous slings, mesh or bulking agent injections who still have incontinence? A subset analysis of Dr. Kaufman’s autologous muscle-derived cell therapy for women with incontinence focused on this group of women. “An Evaluation of Women with Persistent or Recurrent Stress Urinary Incontinence (SUI) Following Surgery in a Double-blind, Randomized, Controlled Trial Comparing Safety and Efficacy of Autologous Muscle Derived Cells for Urinary Sphincter Repair (AMDC-USR) with Placebo (PBO),” a multi-center double blind placebo-controlled study, investigated women with SUI with persistent symptoms despite prior surgery. Patients could have up to five prior surgeries. Over 80% required a mid-urethra sling. “This is the greatest unmet need,” said Michael B. Chancellor, MD, the study’s principal investigator. Dr. Chancellor is director of the neurourology program at Beaumont Hospital in Royal Oak, Michigan.
Subjects were randomized 2:1; 50 women received the cell therapy injection, and 25 received placebo. After 12 months, placebo patients could receive AMDC-USR.
After 12 months, the primary outcome was a greater than 75% reduction in the SIEF. There was significant improvement with AMDC but not with placebo injection.
“Moreover, when going on to open label, the placebo patients who received the cell therapy improved,” Dr. Chancellor said. “For women with prior incontinence surgeries, the use of AMDC-USR treatment was safe and well tolerated with follow up over two years. AMDC-URS has been granted an expedited Regenerative Medicine Advanced Therapy designation by FDA. Phase III trials are ongoing.”
Could exercise reduce the risk of urinary incontinence?
“The Association of Physical Activity and Urinary Incontinence in Women: Results from a Multi-Year National Survey,” set out to identify the relationships between different types of physical activity, both work and recreational, and different types of urinary incontinence among women. The study relied on cross-sectional data from the National Health and Nutritional Examination Survey from 2008 to 2018 from 30,213 women age 20 and older; 23.16% self-reported UUI.
“There’s an inverse relationship between physical activity and all types of incontinence,” said Sanam Ladi-Seyedian, a study investigator and research fellow in the department of urology at the University of Southern California.
Visit AUA2021 Daily News Online for more articles.